Selective CO_{2} fixation reaction to styrene oxide by Ta-substitution of Lindqvist-type [(Ta,Nb)_{6}O_{19}]~{8-} clusters
V. Chudatemiya, M. Tsukada, H. Nagakari, S. Kikkawa, J. Hirayama, N. Nakatani, T. Yamamoto, S. Yamazoe
Catalysts, Accepted.
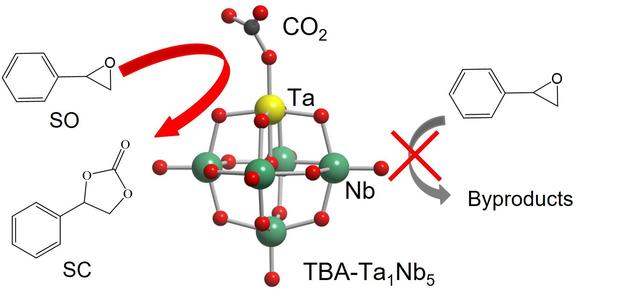
Metal oxide clusters composed of group 5 metal ions, such as Nb and Ta, exhibit catalytic activities for CO_{2} fixation to styrene oxide (SO) due to highly negative natural bonding charge of the terminal O atoms which could work as CO_{2} activation sites. In this study, tetrabutylammonium (TBA) salts of [Ta_{x}Nb_{6−x} O_{19}]~{8-} (TBA-Ta_{x}Nb_{6−x}, x = 0−6) have been prepared and Ta-substitution effect on catalytic properties of TBA-Ta_{x}Nb_{6−x} for CO_{2} fixation to SO was investigated. We found that TBA-Ta_{1}Nb_{5} showed the highest styrene carbonate (SC) selectivity (95%) among TBA-Ta_{x}Nb6−x although the SO conversion monotonously increased with the increment of Ta substitution amount. The CO_{2} fixation reactions into SO under various conditions and in-situ X-ray absorption fine structure measurements revealed that CO_{2} was activated on both terminal O sites coordinated to Ta (terminal O_{Ta}) and Nb (terminal O_{Nb}) sites whereas the activation of SO proceeded on terminal O_{Ta} and/or bridge O sites which were connected to Ta. Density functional theory (DFT) calculation revealed that the terminal O_{Ta} of TBA-Ta_{1}Nb_{5} preferentially adsorbs CO_{2} compared with other O_{Nb} base sites. We concluded that the selective CO_{2} activation at terminal O_{Ta} of TBA-Ta_{1}Nb_{5} without SO activation is a crucial factor for high SC selectivity in the CO_{2} fixation reaction to SO.