Superior Base Catalysis of Group 5 Hexametalates [M_{6}O_{19}]~{8-} (M = Ta, Nb) over Group 6 Hexametalates [M_{6}O_{19}]~{2-} (M = Mo, W)
Shun Hayashi, Naoto Sasaki, Seiji Yamazoe,* and Tatsuya Tsukuda*
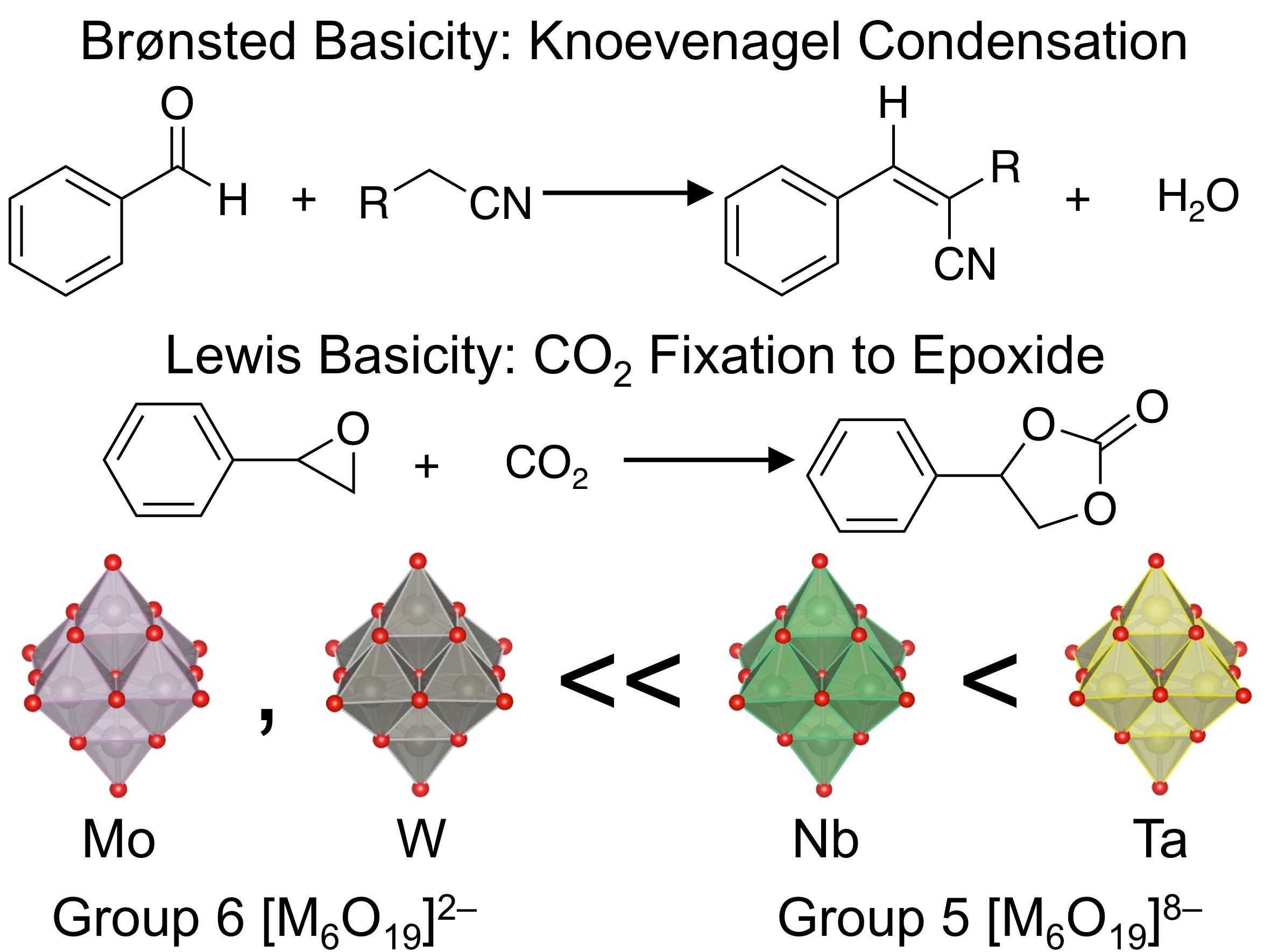
Brønsted and Lewis base catalysis of hexametalate clusters of group 5 metals [M(V)_{6}O_{19}]~{8-} (M(V) = Ta, Nb) and group 6 metals [M(VI)_{6}O_{19}]~{2-} (M(VI) = Mo, W) was studied using Knoevenagel condensation and CO2 fixation reaction, respectively, as test reactions. It was found from mass spectrometry and elemental analysis that tetrabutylammonium salts of [M(V)_{6}O_{19}]~{8-} were partially protonated to form [H_{4}M(V)_{6}O_{19}]~{4-} under ambient conditions, whereas those of [M(VI)_{6}O_{19}]~{2-} were not. Base catalytic activity increased in the order of [Mo6O_{19}]~{2-}, [W_{6}O_{19}]~{2-} << [H_{4}Nb_{6}O_{19}]~{4-} < [H_{4}Ta_{6}O_{19}]~{4-}, which is consistent with the order of the average NBO charges on the surface O atoms. This trend suggests that the highest activity of [H_{4}Ta_{6}O_{19}]~{4-} is due to the large amount of negative charges on surface O atoms. Theoretical calculations on [H_{n}Ta_{6}O_{19}]~{(8-n)-} (n = 1-4) demonstrated that protonation of the O atoms at the edges is energetically favorable regardless of n and that the NBO charges on the remaining unprotonated O atoms in [H_{4}Ta_{6}O_{19}]~{4-} are still more negative than those of pristine [Nb_{6}O_{19}]~{8-}, [W_{6}O_{19}]~{2-} and [Mo6O_{19}]~{2-}. Theoretical calculations also predicted that CO2 can be reductively activated at all the surface O atoms of [H_{4}Ta_{6}O_{19}]~{4-} regardless of their locations. This work demonstrates that group 5 polyoxometalates are promising candidates for active base catalysts.